
A quality audit is a procedure in which a team of auditors, either internal or external, thoroughly examines a quality system. It is a key component of the ISO 9001 quality system standard. Audits are needed to determine whether newly adopted or existing processes, products, and systems successfully deliver the greatest benefit to a business or organization. It is an important feature of any organization's quality management system and is widely used in many industries.
In the manufacturing world, quality audits are an effective way to check that your company or your vendors’ manufacturing activities are meeting your product quality standards. It is also used to check for conformance to your defined QMS quality system rules and guidelines.
The American Society of Quality (ASQ) is the recognised authority on best practices in quality audits and many professional auditors obtain ASQ quality auditor certifications. In general, a quality audit focuses on evaluating the system's performance through an unbiased, systematic and documented process. The auditor evaluates the data and assesses the findings in terms of how well the system or process under audit is working.
Here we'll go into details of quality audits, examining what they are, their types, importance, the steps involved, and how your organization’s manufacturing or sourcing activities may benefit from them.
For companies with product manufacturing, engineering, supply chain, or sourcing activities, quality audits are essential for the following reasons:
If you are working with external vendors, manufacturing partners, and contract manufacturers, quality audits are a key method of benchmarking their performance against your QMS and quality requirements.
What is being audited determines the type of audit. Although there are many different types of quality audits, process, product, and quality system audits(QMS) are the three main categories. We'll go into more depth of these categories.
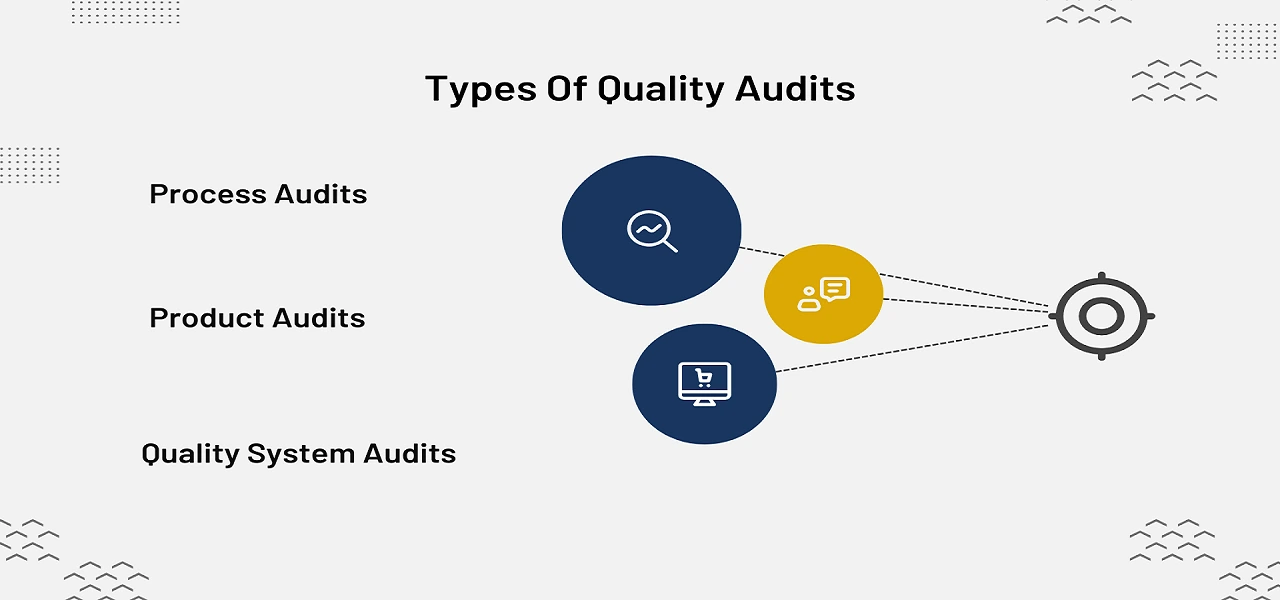
The purpose of this audit is to determine whether a company's procedures adhere to established regulations (such as FDA GMP) or industry standards (such as ISO). By comparing the company's procedures, work instructions, training logs, job descriptions, etc. to the established standards, the auditor will evaluate the process controls. Process audits can be carried out on all of the quality system's processes or just a few of them.
This specific kind of quality audit looks into whether a certain good or service (hardware, processed goods, or software) meets with performance standards or customer needs. To determine whether the product complies with desired product requirements, it will be assessed against performance standards, product specifications, and most probably customer requirements. Product audits are typically carried out during or just after the production process.
This particular type of quality audit will make sure all components of a quality management system (QMS) have been developed, put into practice, and documented in accordance with the necessary specifications and international best practices as defined by the ISO 9001:2015 Quality Management System Standard. By comparing procedures and policies, the auditor will assess the company's quality system to identify conformance levels. Quality professionals recommend that quality management system (QMS) audits must be performed at least once in a year.
These are the key areas of a quality audit:
The standards, laws, and other requirements against which the audit is conducted.
The facts and information gathered throughout the audit process to substantiate conclusions.
Observations, non-conformities, and areas for improvement are all included in the audit findings.
Actions taken by the organization to address discovered non-conformities and stop them from repeating again are known as corrective actions.
To further understand what is a quality audit, let's have a look at the quality auditing process.
Starting the quality audit is the first step in the audit process. To initiate a quality control audit, the customer, the auditee, and the auditing company or auditor have to first communicate with one another so that the audit objectives are fully understood. This forms a point of reference to design the audit framework and parameters
The auditing company’s or auditor’s planning for the audit includes a number of activities from the three parties involved in the audit, such as scheduling the audit, estimating the amount of resources required for the audit, and preparing the audit questionnaire.
Following the quality audit planning, the auditor begins a number of tasks, such as assessing system and process controls, interviewing stakeholders, reviewing technical processes, and doing documentation reviews. These tasks are conducted according to the audit parameters.
The auditor puts together a report of audit findings. The audit report contains performance assessments against the audit criteria and provides independent insights into how the auditee company addresses quality management in their organisation.This report enables an organization to efficiently monitor performance and quality, identify areas for further improvement, and highlight any successes.
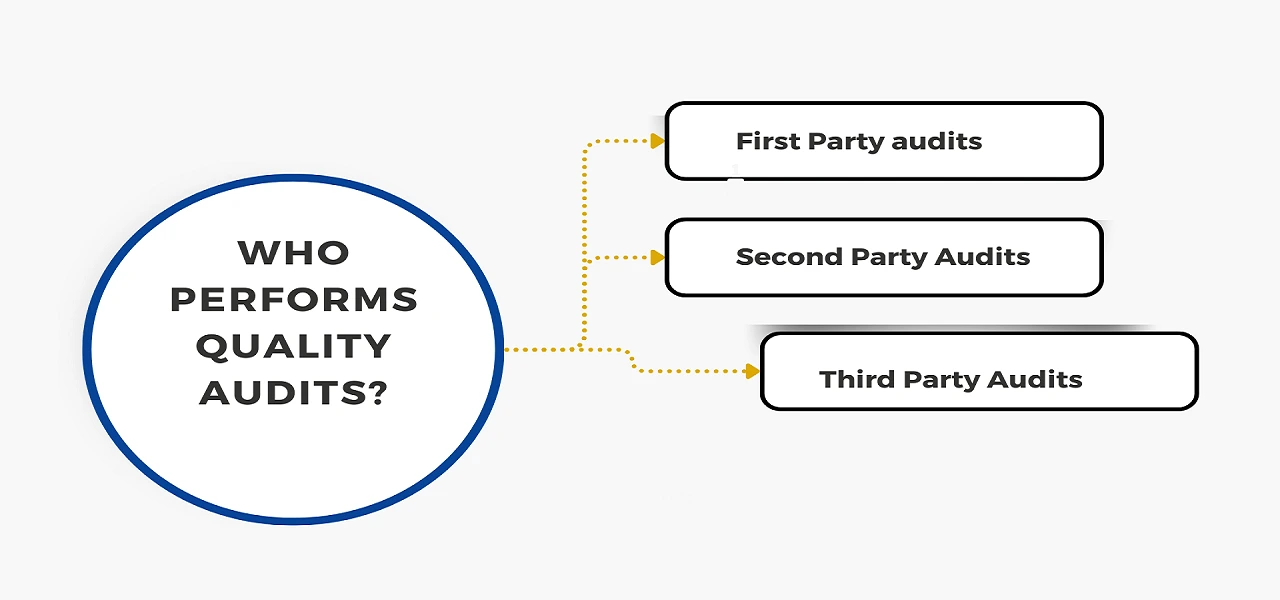
Depending on who conducts the audits and what the audits are intended to achieve, there are distinct audit types.
Quality auditing can add significantly to a company's worth and helps to create a ‘quality first’ culture in your organization, suppliers, and supply chain.
The process can:
Regular quality audits in your organization have a lot of advantages. Here is how they can help your company. Quality audits:
A Quality Audit Checklist is a tool that assists auditors, quality managers, inspectors, or a designated person in auditing products or operational processes.It aids in evaluating how effectively established quality control processes are working and in identifying any underlying problems that call for correction. Regular quality control audits can drive good product quality management practices, increase business efficiency, and enhance corporate strategies while still adhering to current requirements
Quality audit checklists are written according to the company’s audit objectives and may have specific technical areas that need to be reviewed for quality performance. The ISO 9001:2015 Quality Management System standard is often used as a guide for the key areas the quality audit checklist should cover. We have summarized these as follows:
Both QC audits and QA audits utilize the same auditing process used by auditors to determine if products, production processes, and management systems adhere to predetermined criteria.
The statement that only internal auditors can perform quality control audits is wrong. Both internal and external auditors are able to perform quality control audits. The choice of using internal or external auditors is based on the particular requirements and conditions of the organization.
Quality audit failures can be prevented by having professional auditors who have strong investigative skills, technical knowledge, and experience in conducting quality audits within the manufacturing and engineering contexts. They should also have engineering backgrounds - it’s important to check this carefully because many quality auditors may have more of a finance or business background. Being clear about the audit objectives and requirements, having a good audit checklist, can go a long way in ensuring the audit is a success.
A quality audit, facilitated by AMREP’s Quality Assurance Services, enables organizations to enhance their overall efficiency, effectiveness, and commitment to delivering products and services that meet or exceed specified quality standards. So if you haven’t initiated a quality audit yet in your company or on your suppliers, now’s the perfect time to get started!
Need help planning and conducting your audits – contact AMREP Supplier Management Services’ Independent Quality Auditors for professional quality auditing services.